WATER 101:
Understanding Water for Injection (WFI)
What is Water for Injection (WFI)?
Water for Injection (WFI) is the highest purity grade of water used in pharmaceutical and biopharmaceutical manufacturing. It is not just a utility—it is a fundamental ingredient in the production process of countless injectable and sterile drug products.
In sterile manufacturing, WFI serves as a direct component of parenteral drug formulations, where it is used to dissolve or dilute active pharmaceutical ingredients (APIs). Because these products are administered directly into the bloodstream or body tissues, the water must be free from microbial contaminants, pyrogens (such as endotoxins), and chemical impurities. Even the slightest deviation from purity standards could compromise patient safety.
Beyond formulation, WFI plays a critical role in equipment cleaning, component rinsing, and environmental control. It is used to rinse vials, ampoules, and manufacturing vessels, ensuring that no contaminants remain that could degrade product quality or cause patient harm. It is also employed in the final rinsing of clean-in-place (CIP) and steam-in-place (SIP) systems, essential for maintaining aseptic conditions in production lines.
Because of its pervasive use and contact with both product and process, WFI is more than just water—it’s a regulated raw material that must meet the highest global pharmacopeial standards, including stringent controls for conductivity, Total Organic Carbon (TOC), bioburden, and endotoxins. Any breach in WFI quality can trigger costly batch rejections, regulatory citations, or—most importantly—risks to patient health.
The integrity and quality of WFI are therefore central to the manufacturing of safe, effective, and compliant injectable medications, making its production and storage not only a technical requirement but a strategic priority for pharmaceutical manufacturers.
Pharmacopeia Acceptance & Requirements
Different global pharmacopeias provide regulatory frameworks for WFI production:
- United States Pharmacopeia (USP)
Specifies conductivity, TOC, and endotoxin limits, allowing WFI production via distillation or validated membrane-based methods. - European Pharmacopeia (Ph. Eur.)
Historically allowed only distillation; membrane-based production permitted since 2017 under strict validation protocols. - Japanese Pharmacopeia (JP)
Similar evolution as Ph. Eur., transitioning from distillation-only to acceptance of advanced membrane technologies (reverse osmosis/ultrafiltration).
| Ph Eur | USP | JP | |
| Conductivity | <1.1 µS/cm at 20 °C | <1.3 µS/cm at 25 °C* | <2.1 µS/cm at 25 °C** |
| Bacteria | <10 cfu/100 mL (guideline action limit) | <10 cfu/100 mL (guideline action limit) | <10 cfu/100 mL (guideline action limit) |
| Total Organic Carbon (TOC) | <0.5 mg/L*** | <500 ppb | <0.5 mg/L |
| Endotoxins | <0.25 IU/mL | <0.25 IU/mL | <0.25 IU/mL |
*Offline conductivity measurements possible; if inline conductivity exceeds values, refer to USP tables in section 645
**Offline conductivity limit after agitating
***Or pass oxidisable substances test
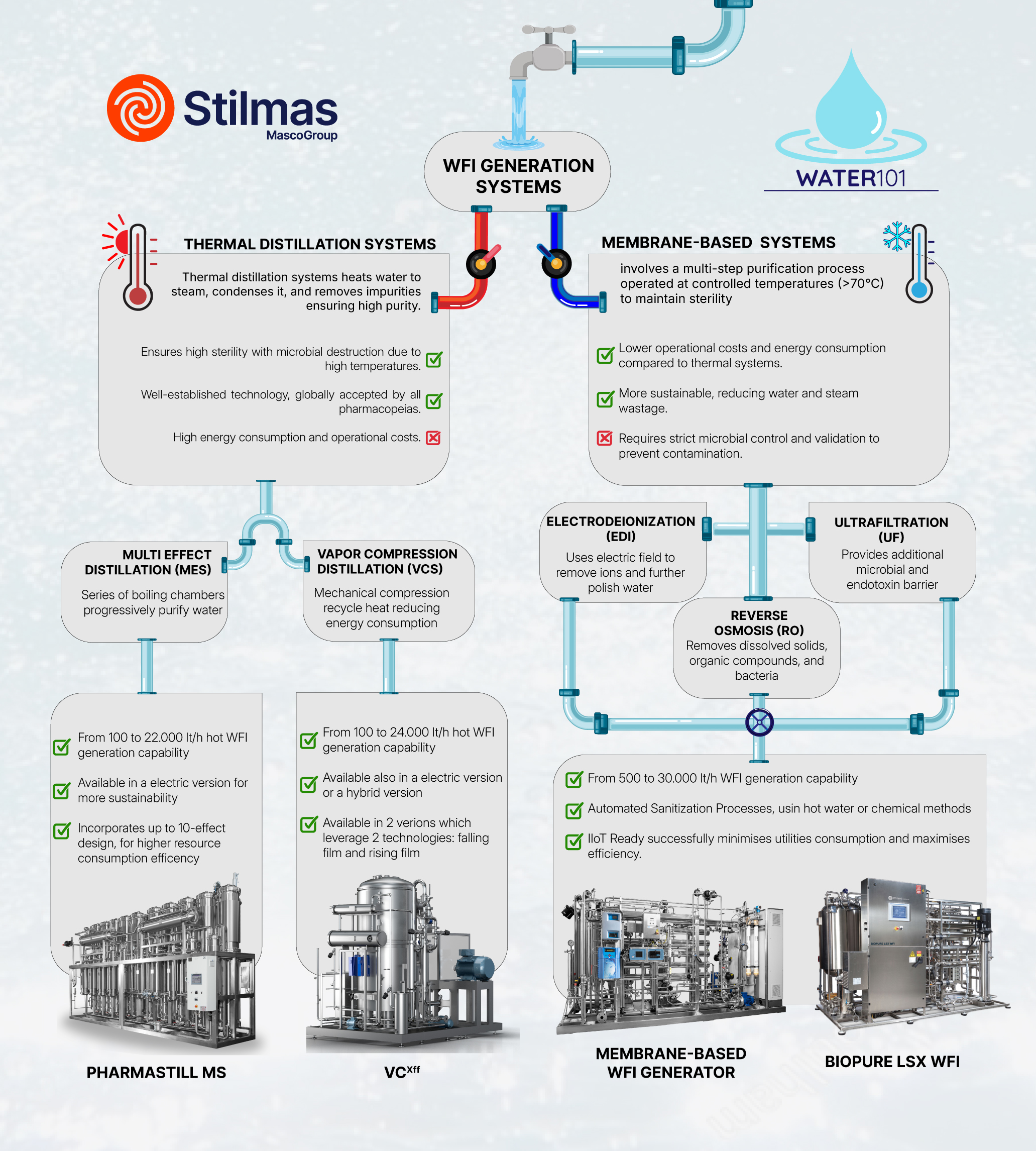
System Designs: Thermal vs. Membrane Technologies
Two principal technologies dominate WFI production:
1. Thermal Distillation Systems
Traditional solutions include Multi-Effect Distillation (MES) and Vapor Compression Distillation (VCS), employing high temperatures to ensure sterility:
- MES: Progressive boiling chambers for incremental purification.
- VCS: Mechanical vapor compression, recycling heat to lower energy demands.
2. Membrane-Based Systems (RO + EDI + UF)
These advanced systems are gaining popularity for their lower energy consumption and operational simplicity:
- Reverse Osmosis (RO): Removes dissolved solids, organic contaminants, and microorganisms.
- Electrodeionization (EDI): Further polishes water to ultra-high purity.
- Ultrafiltration (UF): Provides a critical microbial and endotoxin barrier.
Membrane-based systems typically operate at controlled temperatures (≥70°C) to mitigate microbial risk.
MAINTENANCE CONSIDERATIONS
- Thermal Distillation: Regular descaling, heat exchanger maintenance, boiler inspections; potential wear of steam system components.
- Membrane-Based Systems: Frequent membrane integrity testing, periodic replacements, and rigorous microbial control protocols.
| CRITERIA | THERMAL DISTILLATION | MEMBANE-BASED SYSTEMS |
| CAPEX (Initial Cost) | HIGH (Complex machinery, Large footprint) | LOWER (Simpler installation, Compact footprint) |
| OPEX (Operational Cost) | HIGH (Energy intensive, Higher maintenance) | LOWER (Energy efficient, Minimal maintenance) |
| Energy consumption | HIGH (Steam-driven operations) | LOW (Electrically powered) |
| Water Recovery Efficiency | MODERATE (Water losses) | HIGH (Efficient water usage) |
| Validation Complexity | Estabilished, Straight forward | Requires thorough microbial validation |
Why Choose Stilmas for WFI Generation?
At Stilmas, we offer more than just compliance—we deliver precision-engineered systems that align with your strategic priorities, whether that’s operational reliability, sustainability, or compact integration. With decades of experience and global recognition in the pharmaceutical and biotech industries, we support your path to regulatory confidence and production excellence.
The Right Solution for Every Application
For Robust, High-Capacity Thermal Distillation:
Trust Stilmas Pharmastill MS or Vapor Compressor VCfxx—our flagship thermal systems that ensure time-tested purity through multi-stage distillation.
- Ideal for: High-throughput facilities, sterile injectables production, and markets favoring conservative validation protocols.
Key benefits: Reliable operation, superior bacterial control, reduced validation complexity, and long-term durability under intensive use. These systems offer unmatched sterility assurance and compliance with even the most conservative pharmacopeias, including JP.
For Energy-Efficient WFI Production:
Choose our BioPure LSX WFI or our membrane-based WFI generator—your go-to solution when footprint, sustainability, and flexible output are key.
- Ideal for: Modern biotech facilities, multiproduct manufacturing sites, and greenfield plants aiming for reduced energy costs.
- Key benefits: Reverse osmosis + EDI + ultrafiltration in a single compact skid, automatic hot water sanitization, and customizable flow rates. It meets USP and Ph. Eur. standards while drastically lowering OPEX. Plug-and-play installation reduces downtime and validation burdens.
To watch the infographic in high definition click here:
Ready to Elevate Your WFI Strategy?
Choosing the right WFI solution is more than a technical decision—it’s a strategic one. Whether you’re building a new facility, upgrading legacy systems, or pivoting toward sustainability, Stilmas has the expertise, technology, and service support to get you there—faster and smarter.
Our specialists are ready to help you evaluate your production goals, navigate pharmacopeial requirements, and recommend the best-fit solution: schedule a consultation today and take the first step toward safer, more efficient, and fully compliant pharmaceutical water systems.
Click on the link below — and let’s shape the future of your clean utilities together.


